Course Organization
- Overview of course content
- Instructor
- Schedule and Format
- Grade Determination
- The Book and Resources
- Other Items
Overview and Learning Objectives
This is a one semester undergraduate course in thermodynamics and statistical mechanics. Statistical Mechanics is probably the most difficult, and most interdisciplinary courses in the undergraduate (and graduate) curriculum. It starts with all of physics, and combines this starting point with challenging concepts (at least for me) to make predictions about real things.
The structure and tentative order of the course will roughly follow our text book by Daniel Schroeder, see below. We will also draw from the presentation given Blundell and Blundell's (also excellent) book, see below. But the presentation will be my own and the outline given below reflects my experience teaching the course for the prior two semesters. Lecture notes of what is discussed in class will be provided. Weekly homeworks will be assigned.
- Basic Thermodynamics
- The pressure and energy of and ideal gas. The equipartition theorem.
- The first law and work
- Isothermal and adiabatic expansion engine cyles
- Phase diagrams, latent heat, and enthalpy
- Non-ideal gasses
- Statistical preliminaries
- Basic Combinatorics and the Stirling Approximation
- Probability Distributions
- Sums of random numbers and the central limit theorem
- A first look at partition functions
- The Boltzmann distribution is stated without proof. We use this to derive the properties of two model systems: the two state system and the classical gas
- Kinetics of ideal gasses
- Boltzmann velocity distribution
- Ideal gas law
- Flux through a hole
- Entropy and the second law
- Entropy and its increase
- Counting states
- Model I: the entropy of a state system
- Model II: the entropy of an ideal gas
- Temperature and equilibration of energy
- Energy versus temperature in Model I and II
- Examples of energy exchange and entropy changes
- Pressure and equilibration of volume
- The pressure of an ideal gas from its entropy
- Examples of volume changes and entropy change
- Thermodynamics and free energy
- Free energy and the availability of work
- Energy, Enthalpy, Free Energy, Gibb's Free Energy and Maxwell Relations
- Statistical Mechanics and the canonical ensemble
- The canonical ensemble
- Entropy and free energy from the canonical ensemble
- Ideal gas redo
- Diatomic gas
- Chemical Equilibrium
- The chemical potential and chemical equilibrium
- The grand canonical ensemble
- Equilibrium between species and the Saha equation
- Quantum Gasses
- Fermi and Bose Distributions
- The photon gas
- The degenerate fermi gas
The goal of this course is for you to be able to solve physics problems associated with these topics. The homeworks are designed to help you achieve this goal.
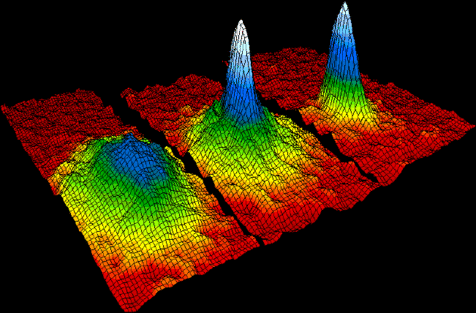
The image below shows the velocity-distributions for a gas of rubidium atoms, indicating the formation a Bose-Einstein condensate. For this and related experiments, Eric Cornell, Carl Weinman, Wolfgang Ketterle received the 2001 Nobel Prize. Left: just before the appearance of a Bose-Einstein condensate. Center: just after the appearance of the condensate. Right: after further evaporation, leaving a sample of nearly pure condensate. We will discuss Bose condensation at the end of the course.
Lecture Instructor:
Professor, Derek Teaney: derek.teaney ![]() stonybrook.edu
stonybrook.edu

Teaching Assistant: Mathieu Boisvert.
The TA will communicate via email, Mathieu.Boisvert  stonybrook.edu
stonybrook.edu
Schedules and Format
The course consists of lectures, homework, midterm and final exams, and office hours.
Lectures, class meetings, and lecture notes
The course consists of two lectures per week
- Lecture Hours: TuTh 10:00-11:20 a.m. in Frey Hall 301
Detailed lecture notes will be given. But, these notes are not a book, and are not a substitute for going to class. My recommendation are to take notes in class
Homework submitted online:
Homework is a significant part of the course. It will be assigned approximately weekly, and students should expect to spend approximately 8-10 hours a week on homework. There will be approximately 12 assignments during the semester.
Homework will be collected online through brightspace. Students will need to produce a scan (of reasonable quality) of their homework, and submit it electronically as a single pdf document. Individual jpegs are not acceptable and will not be graded. For a typical student, the program CamScanner, which can be installed on any modern phone, is a useful tool to scan handwritten pages and convert them to a single pdf document.
Homework will be accepted late, but will be penalized at 2% per day. Homework turned in after ten days will receive up to 50% of the maximum grade.
Exams
The Final Exam:
The Final exam is on Tuesday, May 7, 2024 from 8:00 A.M. -- 10:45 A.M (yikes that is early!) in our class room.
The midterms and quizzes:
The precise date for the Midterms will be announced at a later time. There may be one or more quizzes, depending on timing. These will be announced, and not a "pop" quiz.
Makeup exams:
Written makeup exams will not in general be offered, except at the discretion of the Professor. Makeup exams will be oral exams covering the same topics as the original exam.
Office Hours:
Office hours are 3:00pm on Wednessday either in the the Nuclear Theory Common room (C134) or in my office (C135). You can stop by at other times, but of course I could be busy with other things.
Our TA is Mathieu Boisvert will also hold office hours
Technical requirements:
- Students will need to produce a scan (of reasonable quality) of their weekly homework, and submit it electronically as a single pdf document. Individual jpegsand other formats will not be accepted. For most students, the app CamScanner, which can be installed on any modern phone, is a useful tool to scan handwritten pages and convert them to a single pdf document. This can be submitted to brightspace.
- You will need some kind of calculator for exams.
Grade Determination and Homework
The grading will be based roughly on the following table. I reserve the right to change these proportions (within reasonable limits) as the course progresses to provide the best overall assessment of the class as a whole. My intent of course is to follow these guidelines.
| Homework and Quizzes | 15% |
| Midterm Exam 1 | 40% |
| Final Exam | 45% |
There may be one or two pre-announced (i.e. NOT pop) quizzes.
The Book and Resources
The required book for the course is
- An Introduction to Thermal Physics by Daniel Schroeder. This is a standard book for this course.
In the past I used
- Thermal Physics by Blundell and Blundell. This is also a standard book for this course. It nicely covers probability but then gets bogged down with engine cycles.
Some other books and notes which I used when preparing the course are:
- Fundamentals of Statistical and Thermal Physics by Frederick Reif. This is probably the best undergraduate boook on Statistical Physics, in terms of correctly explaining the underlying physics. However, it veers towards the graduate level and is a bit too mature for most Stony Brook undergraduates.
- Statistical Physics of Particles by Mehran Kardar. This is a nominally a graduate book. But, the level is moderate, the exposition is very clear, and the author is an excellent physicist, making it a boook to turn to for undergraduates, graduates, and researchers alike.
- Heat and Thermodynamics by Zemansky and Dittman. A classic textbook, first published in 1937. As a student I especially appreciated the discussion of experiment. I have the sixth edition.
- Physical Chemistry by Peter Atkins and Julio Paula. A big chemistry book. Thermodydnamics has a lot of applications, and the unfortunately the way we teach it in physics (and the time devoted to it) makes it way too abstract. Reading some of this book can bring the formalism down to earth.
Other Items
Required e-mail communication
Email to your University email account is an important way of communicating with you for this course. For most students the email address is firstname.lastname@stonybrook.edu. It is your responsibility to read your email received at this account.
Student Accessibility Support Center:
If you have a physical, psychological, medical, or learning disability that may impact your course work, please contact the Student Accessibility Support Center, Stony Brook Union Suit 107, (631) 632-6748, or at sasc@stonybrook.edu. They will determine with you what accommodations are necessary and appropriate. All information and documentation is confidential.
Academic Integrity:
Each student must pursue his or her academic goals honestly and be personally accountable for all submitted work. Representing another person's work as your own is always wrong. Faculty is required to report any suspected instances of academic dishonesty to the Academic Judiciary. Faculty in the Health Sciences Center (School of Health Technology and Management, Nursing, Social Welfare, Dental Medicine) and School of Medicine are required to follow their school-specific procedures. For more comprehensive information on academic integrity, including categories of academic dishonesty please refer to the academic judiciary website at http://www.stonybrook.edu/commcms/academic_integrity/index.html
Critical Incident Management:
Stony Brook University expects students to respect the rights, privileges, and property of other people. Faculty are required to report to the Office of Judicial Affairs any disruptive behavior that interrupts their ability to teach, compromises the safety of the learning environment, or inhibits students' ability to learn. Faculty in the HSC Schools and the School of Medicine are required to follow their school-specific procedures.Further information about most academic matters can be found in the Undergraduate Bulletin, the Undergraduate Class Schedule, and the Faculty-Employee Handbook.