1.6 Pressure and effusion
After a change of variables the probability distribution of velocities can be written as distribution particles with the speed , flying with angles and :
| (1.71) | ||||
| (1.72) | ||||
| (1.73) |
where we have recalled Eq. (1.53) for the speed distribution. Thus, the probability of in a specified range is probability of speed in a range , times a probability of angles which are uniformly distributed the sphere, i.e. . The number of particles per volume with speed in and angles in and is given by the probability in Eq. (1.73) multiplied by the the number of particles per volume .
| (1.74) |
Consider a hole of area on the wall of a container containing the gas (see Fig. 1.3). The flux through the hole is defined as
| (1.75) |
From tube geometry in Fig. 1.3,
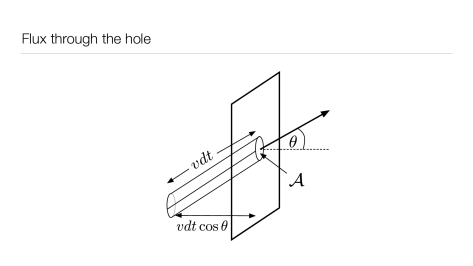
the number of particles flying through the area in time with speed in and angles in and is , where is the volume of the tube and is the height of the tube. Dividing by and we find the differential flux:
| (1.76) |
is the number of particles passing through the hole per area per second with speed in and angles in and .
We can integrate the flux over the velocity and over half of the sphere to find the total flux. Writing the in the as (see homework for motivation), we find:
| (1.77) | ||||
| (1.78) |
We discussed an application or two of Eq. (1.77)
The pressure exerted by the atoms is found by calculating how many particles bounce of the wall per area per time, and the momentum the transfer they impart to the wall. A particle striking the wall velocity , bounces off the wall with velocity delivering an impulse (see Fig. 1.4).
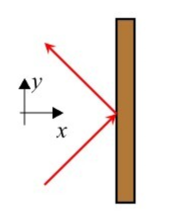
The momentum transfer (or impulse) to the wall by the atom is . (The momentum transfer to the atom by the wall is .) Recall that force is . The momentum transfer per area per per time, or force per area, created by the atoms with speed and angles in the ranges , , is
| (1.79) |
Integrating over as in Eq. (1.77) gives the force per area or pressure. Of course this should give the ideal gas law . Computing the pressure is a matter of integration
| (1.80) |
Using the details are similar to Eq. (1.77)
| (1.81) | ||||
| (1.82) |
It is satisfying how the molecular theory of gasses reproduces the ideal gas law .